O’Neal provides total solution biopharmaceutical projects offering full-service site evaluation, master planning, architectural design, engineering, procurement, and construction capabilities for GMP manufacturing, research and development, process support, facilities, and utilities. Added to these capabilities is in-depth regulatory compliance with FDA and CBER regulations, and cGMP and ISPE best practices.
get connected
Your Representative
Focus Areas:
- Aseptic manufacturing
- Biopharmaceuticals
- Biotechnology
- Next-generation biopharmaceutical facility design
- Legacy facility renovation/expansion
- Biotechnology facility design
- Blood products
- Bulk pharmaceutical chemicals
- Cell + gene therapy
- cGMP fill finish facility design
- Dietary supplements
- Food + beverage
- Industrial biotechnology
- Medical device
- Oral solid dosage
- Parenteral fill/finish
- Parenteral therapeutics
- Pharmaceuticals
- Potent compounds
- Probiotics
- Science + technology
- Vaccines
Special Capabilities:
- Integrated planning, design + construction services geared for regulated life sciences industries for grassroots and existing sites
- In-house design expertise in biotech + pharmaceutical manufacturing processes, bioprocess engineering, regulatory compliance, hygienic/aseptic technology, cleanrooms, HVAC + process utility systems, central and process utilities design
- Long-term industry experience + specialized knowledge, to handle cGMP facility delivery from initial planning + concept development through detailed design, procurement + construction
- Facility delivery for complex, technology + compliance-driven design requirements through full-service capabilities to operate within a cGMP environment
Services:
- Planning
- Site selection support
- Facility programming
- Conceptual design
- Preliminary engineering
- Detailed design
- Process engineering
- Procurement
- Construction
- Process feasibility studies
- GMP equipment design
- Operations improvement
- Process modeling
- Technical consulting
- Operations improvement
Biopharmaceutical WhitePapers

Aligning Objectives for Projects in Legacy Facilities
Dave Marks, PE, O’Neal, Inc.
ABOUT THE AUTHOR: Dave Marks is Director of BioPharm Projects with over 30 years of experience in biotechnology, pharmaceutical, medical device, food and chemical industry projects.
There are two kinds of objectives encountered when renovating legacy manufacturing facilities: opportunity-based and risk-based.
- Projects that are primarily opportunity-driven seek to create a business advantage through facility transformation – usually through improvements in capacity, quality or efficiency. These are the fun projects; the ones most easily justified by return-on-investment, but also the projects that all too frequently never happen because they fall victim to the tyranny of the urgent.
- Projects that primarily mitigate risk are motivated by loss aversion. These projects are primarily driven by an unacceptable business risk associated with people, property or product. These are often the high-profile remediation efforts that jump to the fast track after an accident, loss, or regulatory observation.
Most renovations are not purely opportunistic, or risk-driven. Project objectives typically include a combination of opportunity and risk drivers. Facility renovations are disruptive, so it makes sense to take care of as many issues as you can during construction. However, the objectives of the project, for many reasons, are not always universally understood by everyone on the team.
Without a shared understanding of desired project outcomes, priorities can be confused, owner stakeholders often send mixed signals, and suppliers end up disappointing their clients. A consensus of shared objectives is particularly important when developing a scope of work and is a critical component for decision-making throughout the entire project life cycle.
Project Objectives Identified
As part of Facility Focus, an industry-wide survey of the life sciences industry conducted by O’Neal in partnership with INTERPHEX, industry insiders – those in engineering and operations roles – were asked to rank the most frequent capital project objectives they encounter when renovating manufacturing space. They identified the following most common capital project objectives, listed in order of frequency:
- Opportunity-Based Objectives
- Increase process throughput
- Retrofit for new product or process
- Increase facility flexibility
- Risk-Based Objectives
- Improve product quality
- Achieve regulatory compliance
- Improve reliability
Note that none of the opportunity-based objectives is mutually exclusive with the risk-based objectives. In fact, it’s frequently more efficient to execute projects with multiple compatible objectives under the common overhead of a single project. To do so takes careful planning, and perhaps the willingness to invest in some front-end feasibility analysis and engineering studies to refine the scope. It is very common though. And when this multi-objective project is tied to a schedule and budget, it’s critical that the project priorities are well-understood.
A Problem of Perspective or Perception?
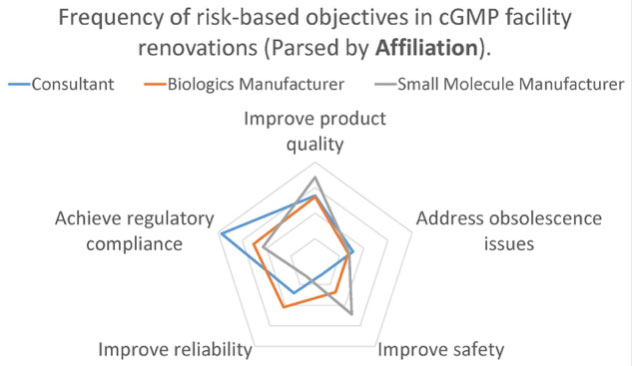
An interesting thing happened when we looked at the survey data broken down by the participant’s affiliation and job function. We were seeing a divergence in terms of the most common reported project objectives. For example, when we asked survey-takers to rank the frequency of risk-based capital project objectives in cGMP facility renovations, we saw a significant divergence in the responses. The radar chart below illustrates the data parsed by the survey participant’s affiliation.
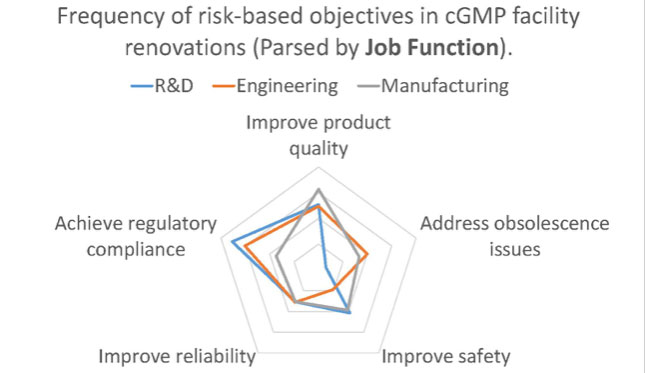
When the same data is parsed by the respondent’s job function, we saw a different frequency distribution.
 Some of these deviations made sense when you considered the perspective of the participants. For example, it’s entirely plausible that participants from an API chemical manufacturing operation (small molecule), which typically involves processes with flammables and combustibles, would more frequently encounter projects in which safety improvement was an objective.
Some of these deviations made sense when you considered the perspective of the participants. For example, it’s entirely plausible that participants from an API chemical manufacturing operation (small molecule), which typically involves processes with flammables and combustibles, would more frequently encounter projects in which safety improvement was an objective.
Likewise, it’s not surprising that those from an R&D background, where the central mission is to develop new products and processes, were less likely to encounter capital projects addressing obsolescence issues. However, other deviations were not as easy to explain.
Why would those working in engineering roles be less likely to encounter projects where safety improvement is a major objective? Why would those representing consultants be more likely to engage in projects where GMP compliance is the primary objective than those who are engaged in GMP manufacturing? In these cases, the divergence in response is most likely due to a difference in perspective.
Those in manufacturing roles are incentivized to see safety improvements as a key objective (it’s their safety we are assuring after all), while those who are supporting manufacturing in engineering roles are more likely to see safety as due diligence, rather than a key project goal. Likewise, those who work for manufacturers are more likely to overlook project objectives related to GMP compliance because they aren’t used to thinking about facility design in that context (in their day-to-day experience they think of GMP requirements in terms of its demands on operations).
But those who design GMP facilities for a living are always thinking about the impact of GMP compliance on facility design, process segregation and product protection (hence the perception that achieving GMP compliance is the foremost objective for these kinds of projects). I’m not saying that the survey reflects a cohort’s conscious choice to pursue different objectives, though stakeholders can and sometimes do advance a personal agenda. What I am saying is that we may be seeing a difference in perception. Our personal perspective – the context of our job responsibilities and relationships – can influence the way that we perceive and prioritize project objectives.
Leveraging Diversity While Aligning Priorities
Fielding a project team with diverse perspectives is good business practice, precisely because of the aforementioned difference in perspectives. This diversity ensures that all the needs of the organization are taken into account when project objectives are first established and helps to identify unintended consequences of choices made throughout the project.
However, the diverse roles and affiliations of project stakeholders also make it harder to establish a shared understanding of primary and secondary objectives, and to maintain these priorities throughout the life of the project.
This is where leadership is required from the project manager. Project managers should be the strongest champions of the project objectives. It is the project manager’s job to ensure that every aspect of the effort – from design to delivery – serves the desired outcome. They are responsible for casting the vision for the project and aligning the team’s efforts in a common direction so that everyone stays on mission.
This leadership starts with a clear articulation of all objectives and priorities, including schedule and budget, from the beginning of the project. Project objectives should be clearly stated in the project plan, in presentations to management, in requests for proposal from suppliers, and most importantly in the kick-off meeting with the project stakeholders. These objectives should be revisited periodically throughout the project, particularly during periods when key design decisions are made.
When difficult decisions need to be made that balance competing priorities, this is the project manager’s compass for determining the right path forward. It’s time to remind the team of their shared vision and get everyone pulling in the same direction. Keeping the project team in sync with these priorities will go a long way toward assuring the success of the effort – as defined by the achievement of all project objectives.
The Facility Focus survey provides insight into the minds of industry insiders – those who have their share of war stories from experience with cGMP projects in legacy facilities.

Design Approaches of GMP Facilities
Dave Marks, PE, O’Neal, Inc.
ABOUT THE AUTHOR: Dave Marks is Director of BioPharm Projects with over 30 years of experience in biotechnology, pharmaceutical, medical device, food and chemical industry projects.
A critical early decision is determining what aspect of manufacturing flexibility is most important before initiating facility design. Surveys we have taken on this topic indicated a clear correlation between an individual’s job responsibilities and how they prioritize flexibility goals. Respondents tended to value that aspect of flexibility that is most useful in their role. Acknowledging that this is a natural human inclination, the survey findings underscore the importance of clearly defining project goals from the onset and consistently reinforcing them with the project team throughout execution.
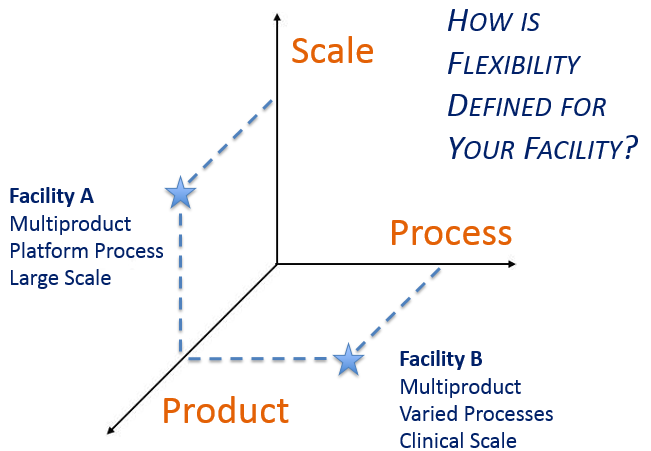
This led to thinking about the dimensions of flexibility within a GMP facility, and how flexible design solutions differ depending upon the manufacturing mission of the facility.
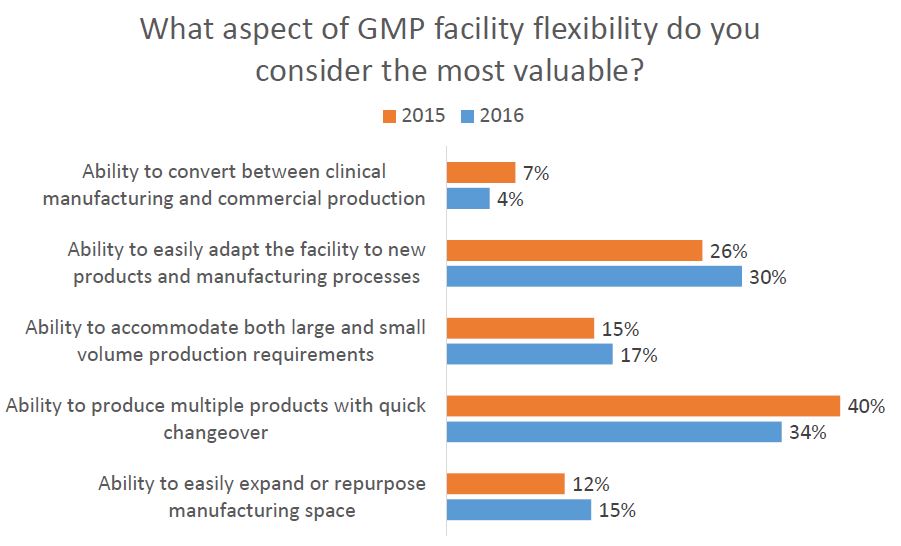
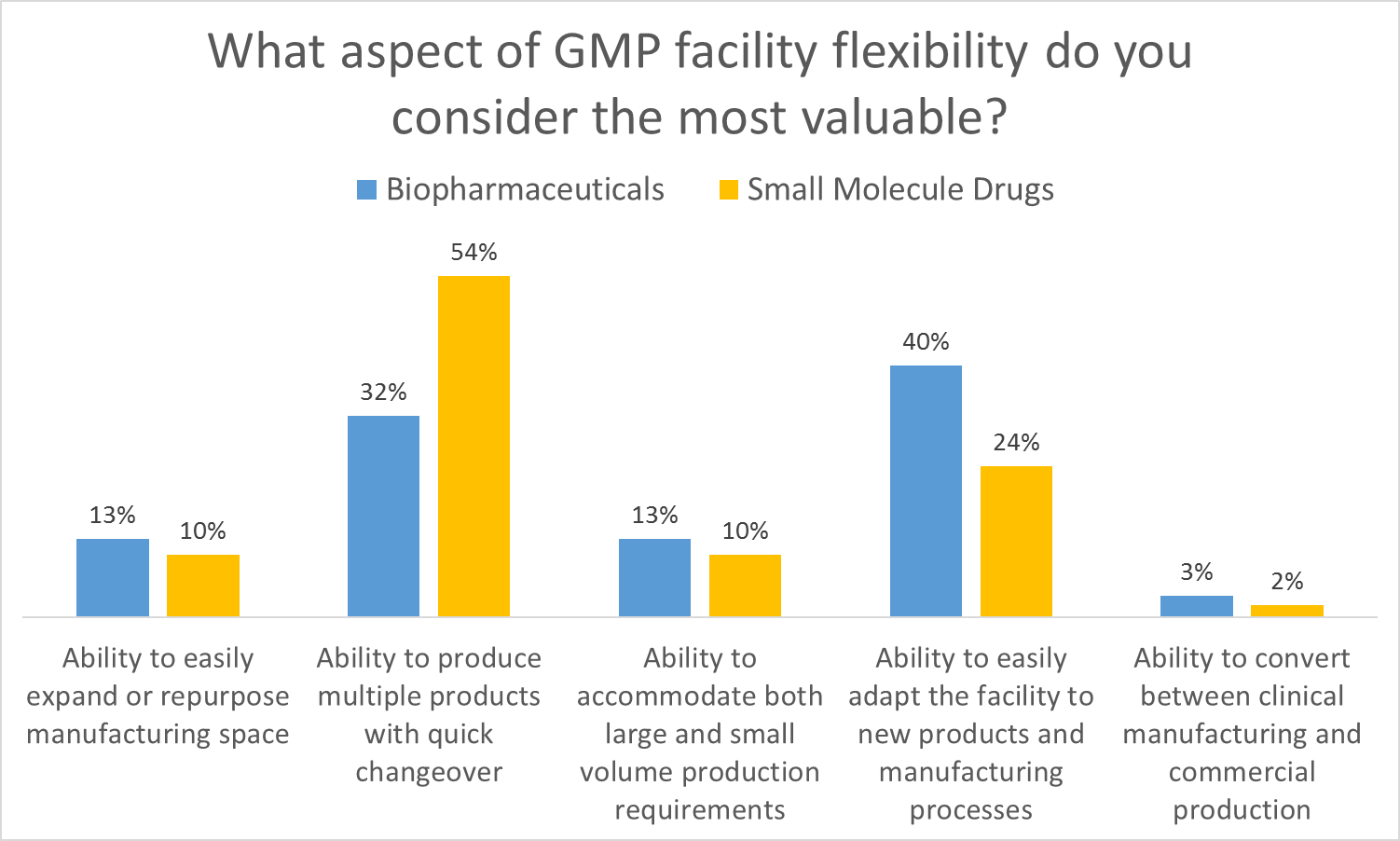
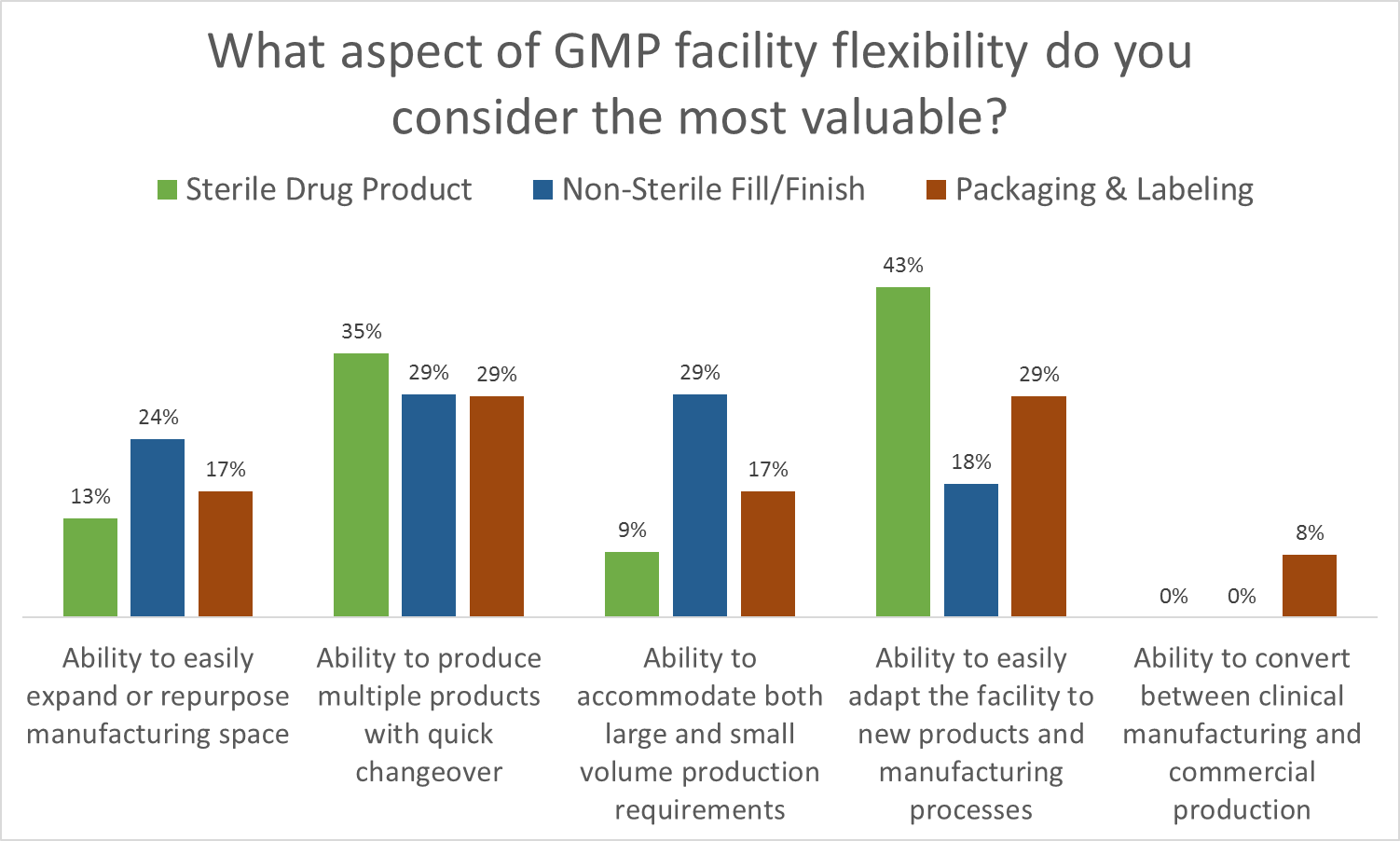
As part of Facility Focus, an industry-wide survey of the life sciences industry conducted by O’Neal in partnership with INTERPHEX, industry insiders – those in engineering and operations roles – were asked what aspect of facility flexibility is most valuable. Survey results were consistent year after year: respondents most highly value the ability to produce multiple products with quick changeover, followed by the ability to easily adapt the facility to new products and manufacturing processes. The strongest preference was expressed by those working in drug substance facilities.

Flexible Biomanufacturing Facilities of the Future Will Impact You
Dave Marks, PE, O’Neal, Inc.
ABOUT THE AUTHOR: Dave Marks is Director of BioPharm Projects with over 30 years of experience in biotechnology, pharmaceutical, medical device, food and chemical industry projects.
Programming GMP facilities has always been a challenging endeavor. Market uncertainties, limited process definition, and stringent regulatory requirements typically complicate the task. Too often, manufacturers end up with facilities that are underutilized or over capacity from day one. McKinsey & Company identifies Rightsizing Capacity and Greater Flexibility as two of the leading trends shaping the landscape of biopharma manufacturing today.
These are really two heads of the same coin, as both elements have a direct impact on facility utilization and cost of goods sold. Generally, facilities that are more flexible tend to be better utilized. But what kind of flexibility is most useful to achieve this utilization? And what are the challenges and trade-offs encountered when we implement new technology to achieve this flexibility?
Industry Insights on Facility Flexibility
As part of Facility Focus, an industry-wide survey of the life sciences industry conducted by O’Neal in partnership with INTERPHEX, we asked industry insiders – those in engineering and operations roles – what aspect of facility flexibility is most valuable. Ranked from most to least valuable, participants responded as follows:
- Ability to produce multiple products with quick changeover
- Ability to easily adapt the facility to new products and manufacturing processes
- Ability to accommodate both large and small volume production requirements
- Ability to easily expand or repurpose manufacturing space
- Ability to convert between clinical manufacturing and commercial production
All of these elements are useful to maximize facility utilization, but overall multiproduct manufacturing with quick changeover is clearly valued the most. This held true even when we broke down responses by job function and affiliation. However, there were some notable differences in response when we looked at the secondary factors:
- Respondents in engineering roles significantly favored the ability to easily expand or repurpose manufacturing space.
- Respondents in manufacturing roles significantly favored the ability to easily adapt the facility to new products and manufacturing processes
- Respondents in R&D roles significantly favored the ability to accommodate both large and small volume production requirements
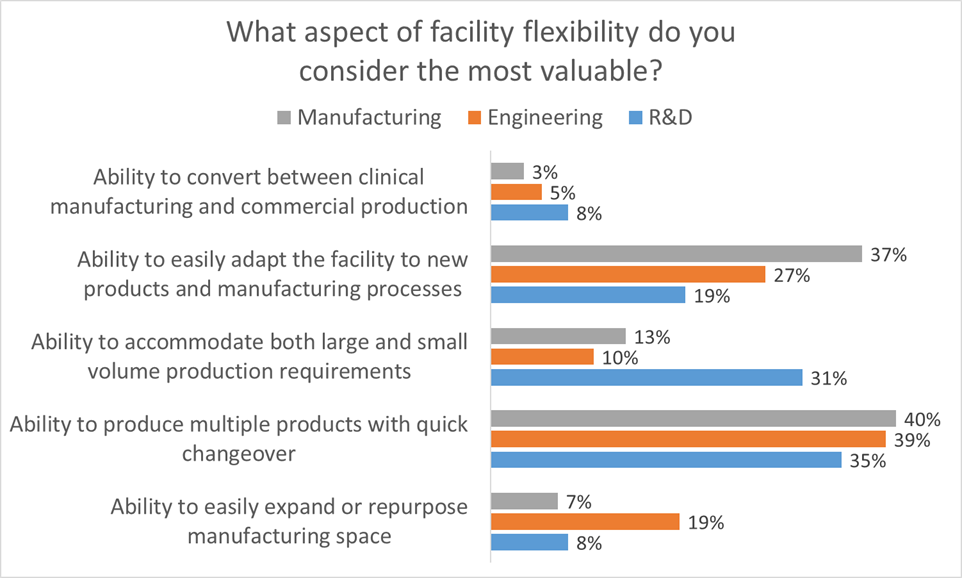
When you think about it these responses make sense because each survey cohort values the aspect of flexibility that is most useful in their role. This helps us to identify the most important aspects of facility flexibility to keep in mind when designing a facility. Traditional approaches to GMP facility design often tend to focus too early on physical attributes such as square footage or process scale as a function of single-product throughput. This approach neglects the foundational dialog regarding flexibility, proceeding on the assumption that all stakeholders understand and agree on the requirements for the facility.
Simply saying that facility flexibility needs to be maximized is inadequate. What is required is a better understanding of the types of flexibility that manufacturers need, from which we can more accurately develop facility designs tailored to the desired outcome. As applied to right-sizing facility capacity and building in flexibility by design, an in-depth grasp of the manufacturer’s business requirements and specific flexibility needs is essential.
Single Use Technology as Disruptive Innovation
The agent most frequently cited as enabling facility flexibility in the biopharmaceutical marketplace is disposables, or single use technology (SUT). This is because SUT conveniently allows manufacturers to outsource one of the most difficult aspects of multi-product bioprocessing – achieving process closure that fully segregates the product from potential sources of contamination, including cross-contamination from other products.
In so doing, it helps to realize the potential for licensed facilities to manufacture multiple GMP regulated products – a much more flexible paradigm. SUT also significantly reduces the time required for product changeover, effectively expanding plant utilization. Finally, SUT provides a tool for reducing the cost and complexity of facilities required for biopharmaceutical manufacturing. When applied comprehensively, SUT can lower the capital cost by reducing reliance on cleanroom environments to provide product segregation and eliminating the facility infrastructure required for clean-in-place and steam-in-place operations.
SUT is sometimes referred to as a disruptive technology. Yet there is nothing intrinsically disruptive about SUT. This is why Clayton M. Christensen, who first coined the term “disruptive technology,” now prefers the term disruptive innovation to describe this phenomenon. Christensen recognizes that it is the business model, not the technology per se, that transforms the marketplace. According to the Christensen Institute, “Disruptive innovation describes the process by which technology enables new entrants to provide goods and services that are less expensive and more accessible, and eventually replace—or ‘disrupt’—well-established competitors.” Disruptive innovation is further described as having three ingredients: an enabling technology, an innovative business model, and a coherent value network. The success of single use is an excellent case study in how these three elements need to come together in order to have a disruptive influence.
Like many enabling technologies, single use is surprisingly pedestrian. Those of us who grew up using Hefty bags to take out the trash can attest that there is nothing particularly innovative about the idea of disposable bags for bioprocessing. In fact, disposables were used in bioprocessing for over two decades before the technology began to be associated with the “facilities of the future” trend. One could argue that the development of 0.2-micron disposable sterile filters was a more intrinsically disruptive technology because it quickly enabled mammalian cell culture to displace microbial fermentation as the preferred commercial production platform for therapeutic proteins.
What single-use technology needed to become truly transformative was an innovative business model and a coherent value network. The Christensen Institute describes the type of innovative business model required as one that “targets non-consumers (new customers who previously did not buy products or services in a given market) or low-end consumers (the least profitable customers).” Further, “this is most easily accomplished by new entrants since they are not locked into existing business models.” The pharmaceutical industry provides a textbook example of this principle. Conservative big pharma was never going to be an early-adopter of SUT as a comprehensive business model. It was the small biotech firms – the 2nd and 3rd tier biomanufacturers for whom the cost and time required to build traditional facilities was a significant barrier to entry – these were the non-consumers for whom the “disposable factory” provided a new and viable solution. Top tier biopharm manufacturers studied SUT and tactically introduced the technology into existing biomanufacturing, but they didn’t make a strategic commitment to developing single use facilities until the business model was proven and they realized that they might be at a competitive disadvantage.
The final component, a coherent value network in which upstream and downstream suppliers, partners, distributors, and customers are better off when the disruptive technology prospers, is being realized today. The coherence of this value network is probably best illustrated by the rise of the Bio-Process Systems Alliance (BPSA) as a growing advocacy for single use. Founded in 2005, BPSA describes itself as “an industry-led corporate member trade association dedicated to encouraging and accelerating the adoption of single use manufacturing technologies used in the production of biopharmaceuticals and vaccines.” We now have a network of suppliers, partners, distributors, and customers who are significantly invested in making the single use facility of the future work. This will accelerate the transformative influence that it has on our industry.
Realizing the Benefits of Facility Flexibility
Recognizing that comprehensive single use biomanufacturing is a disruptive innovation, Shire and Biogen were among the first leading biopharmaceutical firms to use this business model to their competitive advantage. The subsequent adoption of the single use paradigm by big pharma validates the success of single use as a transformative influence in our industry. However, the introduction of new technology with a relatively immature supply chain in cGMP facilities is not without it’s difficulties. As part of O’Neal’s Facility Focus survey, we asked biopharmaceutical manufacturers to identify the most significant challenge associated with manufacturing using SUT. The responses revealed just how complex and dynamic this process is. While compatibility was cited as the most significant challenge, it was only a top concern for 25% of the participants. In addition, it is not clear if respondents were thinking in terms of compatibility with the process or compatibility between single-use components (i.e., a lack of industry standardization), so this response could be indicating two very different areas of concern. Several other issues prompted a double-digit response, including cost (18%), supply train (18%), and integrity issues (15%). A significant number of respondents (17%) indicated that their chief concern stems from other issues such as operator mindset, training, waste disposal and warehouse space for consumables.
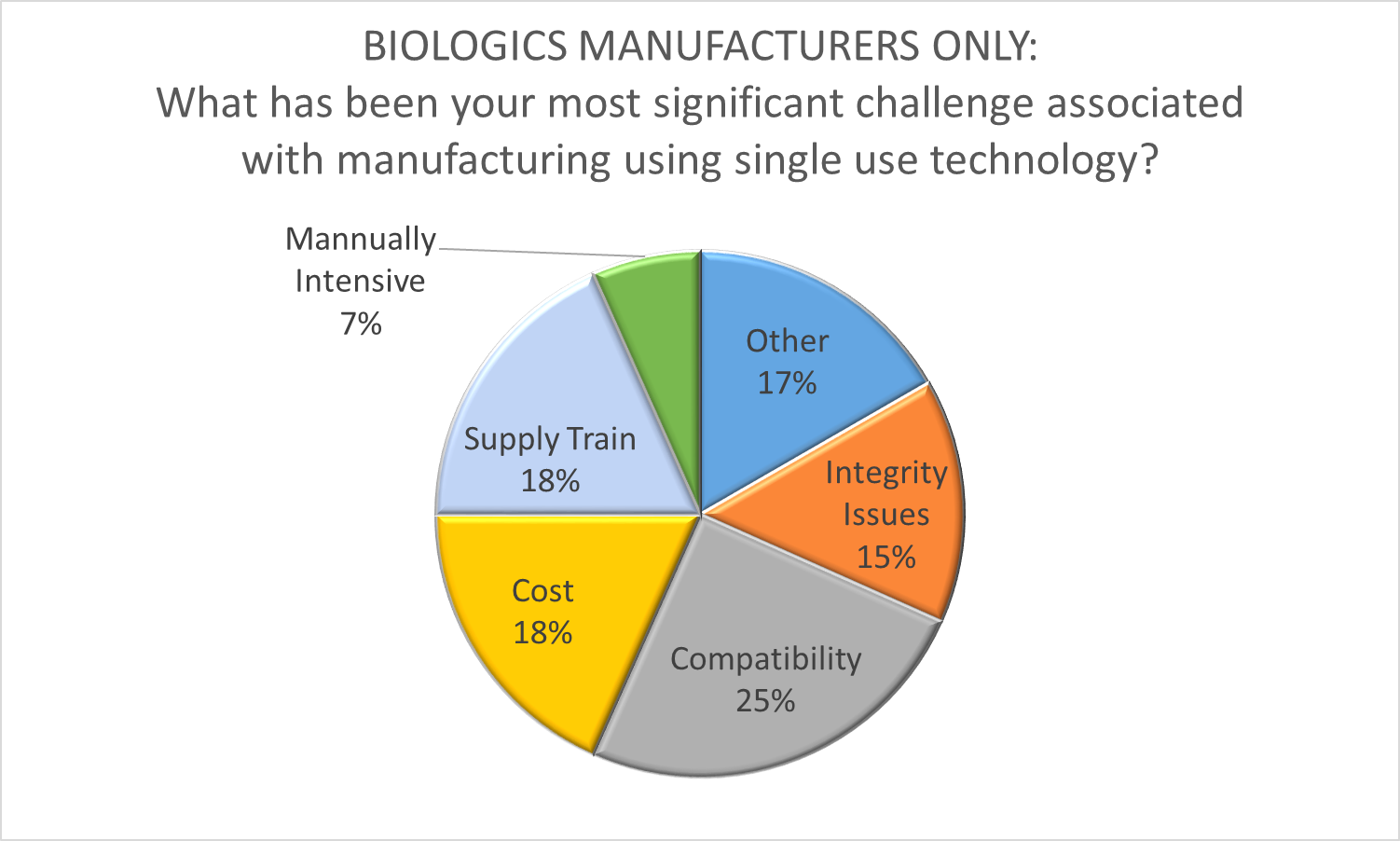
Clearly, there is still much work to be done to realize the full potential of SUT to revolutionize biomanufacturing. It’s just as clear though, that the disruptive innovation genie is out of the bottle – leaving manufacturers scrambling to apply this technology in the most productive way. We’ve had a front-row seat for this industry transformation at O’Neal, having assisted many manufacturers with the early adoption of single-use technology, the development of cGMP facilities designed to realize it’s benefits, and even the application of SUT for production of medical countermeasures by the US government.
We expect the trend to continue to transform biomanufacturing, and merge with other enabling technologies such as isolators and continuous processing, to develop facilities of the future that look very different from the established biomanufacturing network today. This will be coupled with innovative business models for the production of niche biopharmaceuticals, biogenerics, and gene and cell therapy, resulting in the development of a much more flexible and efficient manufacturing infrastructure in facilities of the future.

The O’Neal Facility Focus survey has provided insight into the minds of industry insiders – those who have their share of war stories from experience with cGMP projects in legacy facilities. Our intent at O’Neal is to continue the dialog, culminating in an updated and improved Facility Focus program at the next INTERPHEX.

Industry Feedback on GMP Facility Renovations
Dave Marks, PE, O’Neal, Inc.
ABOUT THE AUTHOR: Dave Marks is Director of BioPharm Projects with over 30 years of experience in biotechnology, pharmaceutical, medical device, food and chemical industry projects.
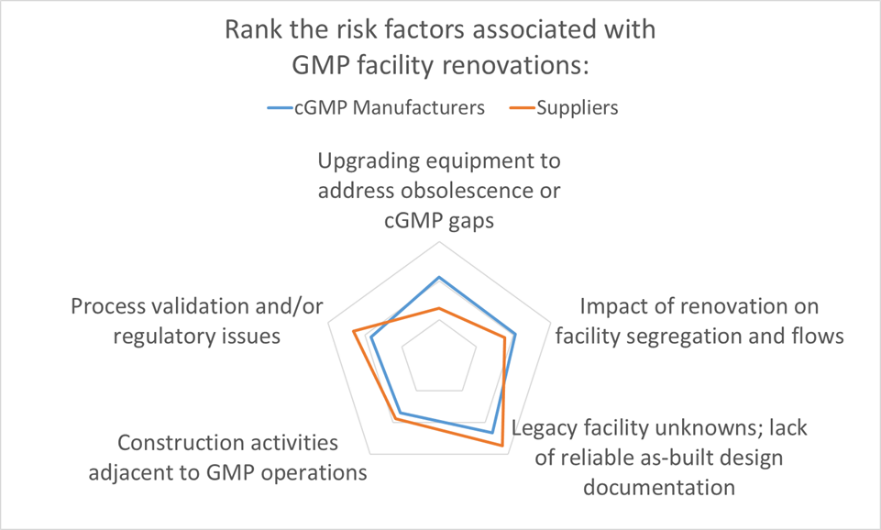
The results are in. As part of Facility Focus, an industry-wide survey of the life sciences industry conducted by O’Neal in partnership with INTERPHEX, we asked industry insiders – those in engineering and operations roles – to rank the risks that they encounter when renovating GMP space. The results were plotted on a radar chart to illustrate the relative weighting that pharma manufacturers and suppliers (mostly consulting engineers) placed on each risk factor.
The Devil You Don’t Know
Survey respondents ranked legacy facility unknowns and lack of reliable as-built design documentation as the leading risk associated with facility renovations. This was not surprising, as anyone with significant experience renovating manufacturing space will have a boatload of stories about projects that went south because of unanticipated issues with the existing space.
Many of the renovation projects we work on at are build-outs of acquired brownfield properties that were originally used for non-GMP purposes. Frequently, the architectural and MEP drawings of such buildings are incomplete, if they exist at all. It is advisable to execute a prospective engineering assessment of a property to determine its suitability for planned GMP operations before committing to its acquisition. Beyond that, some selective demolition and foundation core sampling/testing may be helpful to see what you are up against.
Even when the renovation is in a building that was originally built by the manufacturer, successive renovations and maintenance fit outs frequently leave the engineering documentation fragmented. Legacy piping, ductwork and electrical infrastructure may be abandoned in place, creating a congested mechanical space that is difficult to sort out. The true picture can only be pieced together with help from personnel who were involved in prior facility modifications – people who may or may not be available to assist the project.
Dealing with Unknowns
In any event, the design process for renovation projects typically starts with a field survey to document existing conditions at the site. Even when “as-built” design drawings are available, it is a good idea to verify the accuracy of these drawings during the site survey. At the end of the day, there is only so much you can do to protect yourself against the devil you don’t know. The best approach for GMP space is to commit the resources necessary to maintain drawings in an as-built state. However, many manufacturers fall short of this ideal, and when the renovation site is repurposed from non-GMP space – all bets are off. Lacking good documentation, the best you can do is gather as much data as you can in the beginning of the project and allow some reasonable slack in the schedule and budget to deal with the unknowns.
It’s Complicated
From the GMP manufacturer’s perspective, the other risks queried in the Facility Focus survey were equally challenging. After facility unknowns, the next highest risk identified by GMP manufacturers was upgrading equipment to address obsolescence or cGMP gaps. Suppliers (mostly engineering consultants) ranked this one lower – perhaps because this is an issue that is frequently addressed directly by the owner and equipment vendor – so GMP manufacturers may have a better appreciation of just how difficult this issue can be. The documentation typically provided with proprietary equipment is not intended to support future equipment modifications, and many equipment vendors are not geared for providing custom modifications of legacy equipment. New equipment solutions may be required if the original equipment cannot be modified. If the equipment in question is part of a validated manufacturing process, the task is even more complicated. The feasibility of equipment modifications is best addressed separately and before tackling a facility renovation.
Design to Comply
The remaining risk categories ranked highly in the survey all touch on requirements integral to current Good Manufacturing Practice. In order of survey ranking, these were:
- Impact of renovation on facility segregation and flows
- Process validation and/or regulatory issues
- Construction activities adjacent to GMP operations
Impacts of renovation on facility segregation and flows is a concern, since adding onto or repurposing space often disrupts the orderly movement of people, materials, equipment, product, and waste through the facility. Facility programming is not just focused on segregation and flows for new products or processes; it may require rethinking the movement of people and materials for legacy manufacturing within the facility as well.
It’s no surprise that Process validation and/or regulatory issues also ranked highly, since the central mission of a cGMP project is to ensure manufacturing operations can produce a compliant product. One unique aspect of working in GMP space is that the impact of validation – on cost and schedule – is a significant consideration in the development of the project scope and may place additional constraints on the project.
Finally, most of the renovation projects for which we are engaged involve construction adjacent to ongoing cGMP operations. Minimizing disruption to these operations is always a project mandate. It is essential that any atypical activities within the facility are properly segregated to prevent product contamination. Fortunately, this is a risk that can be mitigated through careful planning. We typically work closely with the owner, building contractors and construction manager to develop a detailed plan for segregating manufacturing space and utilities in operation, and for managing the separate and safe movement of people, equipment and materials to and from the construction zone.

The O’Neal Facility Focus survey has provided insight into the minds of industry insiders – those who have their share of war stories from experience with cGMP projects in legacy facilities. Our intent at O’Neal is to continue the dialog, culminating in an updated and improved Facility Focus program at the next INTERPHEX.

Renovating Your Legacy Facility for cGMP Space
Dave Marks, PE, O’Neal, Inc.
ABOUT THE AUTHOR: Dave Marks is Director of BioPharm Projects with over 30 years of experience in biotechnology, pharmaceutical, medical device, food and chemical industry projects.
Dave Marks, PE, O’Neal, Inc.
Renovating facilities of any kind is tricky business. There are the unknowns for one thing: the patchy documentation; the gotchas behind the walls or under the slab; the awkward fit of repurposed space within existing structures. Then, you have to determine what’s salvageable and how to integrate new construction within the legacy building. Repurposing old facility space can be far more challenging than designing greenfield facilities with the luxury of starting with a clean sheet.

How cGMP + Legacy Facilities is a Game-Changer
Renovating for GMP space is an entirely different ball game. Manufacturing facilities typically have to minimize downtime, requiring a realistic strategy for segregating construction activities in adjacent space and preparing existing utility systems for expanded utilization. The original facility adjacencies will be disrupted, requiring thinking through the impact on supporting operations – warehousing, weigh/dispense, QA labs, solution preparation, equipment cleaning/sterilization, gowning and locker rooms. Consider how the throughput from new or expanded operations will affect these support spaces. The flow of people, materials, equipment, product and waste through the facility will likely be disrupted as well. A well-planned facility expansion or renovation will guard the integrity of GMP traffic and cleanroom transitions for both new and remaining operations. Bottom line, the space program is not limited to the user requirements for the new space; the impact on existing operations must be understood.
Process Operations: Are You Asking the Right Questions?
There are abundant issues associated with legacy process systems as well. Will new equipment be required, or can existing systems be adapted to new process requirements? How will new process systems tie into existing clean utilities and CIP infrastructure? How about tie-ins to existing data historians, SCADA, MES and BAS systems? Are process operations open, closed, or a mix? What is the impact on the degree of facility segregation that required to protect product quality? What are the utility requirements for the new space? How will its load diversity affect other manufacturing operations within the facility? What approach will the renovated facility take to cleaning and bioburden control? These are just some of the many process-related questions that need to be answered as part of scope definition for the project. This underscores the importance of understanding new and existing process operations before committing to a project scope, budget and schedule.
The objectives of the project often necessitate a process-oriented approach to cGMP renovations and expansions. If process remediation is one of the objectives of the project, one needs to take a deep dive into the manufacturing complexities to understand the root cause of issues. If the objective is to expand throughput, then the material balance and timing of operations must be well understood for each product before modeling the process and identify bottlenecks for each scenario. And of course, anytime any disruption to an existing GMP manufacturing operation requires an understanding of the impact on process validation as part of decision-making. Is this a new manufacturing process? How then will the team address interaction and degree of separation required to prevent cross contamination with other products. These are just a few examples of how projects in legacy pharmaceutical facilities are unique.
The Necessity of a Different Mindset
It’s clear that GMP renovations require a different mindset for everyone on the project team – the owner, architect, engineer, construction manager, C&Q team – to ensure a successful outcome. This is where working with an experienced team with the right skillset really pays off. There’s no substitute for a project team that is properly equipped to anticipate the challenges ahead and address them in the project execution plan.

Pharma 4.0: How AI and ML are changing the landscape of the biopharmaceutical industry
Dave Marks, PE, O’Neal, Inc.
ABOUT THE AUTHOR: Dave Marks is Director of BioPharm Projects with over 30 years of experience in biotechnology, pharmaceutical, medical device, food and chemical industry projects.
Bridge Automation is an O’Neal company that specializes in data science, AI and machine learning to improve manufacturing processes and outcomes.
What is Pharma 4.0?
Pharma 4.0, which originated in 2017 through ISPE, is a comprehensive manufacturing approach derived from Industry 4.0. It encompasses the industry 4.0 operating model, focusing on its application to the production of medicinal products. Pharma 4.0 streamlines and optimizes manufacturing processes through digitalization, which leads to increased efficiency, reduced production time, and minimized waste, ultimately enhancing overall productivity.
Digitalization
At present, in most organizations, frequent analytical characterization and quality testing of the intermediate drug substance and final drug product at various points of the manufacturing process are unorganized, scattered in different computer locations, and handled by different teams. This lack of cohesion and integration hinders efficiency, data accessibility, and decision-making capabilities within the pharmaceutical industry.
To address these challenges and embrace the transformative potential of Pharma 4.0, organizations need to take proactive steps. As a pharmaceutical organization, your first step towards Pharma 4.0 is assessing your digital maturity. By evaluating your organization in six different areas—computerization, connectivity, visibility, transparency, productivity, and adaptability—you can identify areas for improvement and chart a path toward digital transformation. Remember that digitalization won’t happen overnight, however, by taking simple yet meaningful steps, you can gradually move closer to Pharma 4.0.
O’Neal’s Project Development and Delivery
Quality Control
Continuous monitoring and real-time handling of deviations
ML-powered techniques can analyze existing data in pharmaceutical manufacturing facilities to monitor the impact of material property discrepancies, procedure variations, and process anomalies like sensor drift and environmental conditions fluctuations that may affect the final product. Continuous monitoring is particularly beneficial in sterilization processes, such as steam sterilization or autoclaving, which are essential for ensuring product sterility. Monitoring variables like temperature,
pressure, and exposure time ensures effective sterilization and compliance with regulatory requirements.
While condition monitoring isn’t new for operations like sterilization, historical data including process parameters in batch operations can help establish a baseline for normal operating conditions, and by comparing this baseline of critical process parameters during operation, deviations can be detected. This approach enables more accurate and earlier detection of anomalies compared to the traditional method of setting alarms based on system variable limits. Other processes that could also benefit from continuous monitoring and detecting a deviation in their batch operations include Cleaning in Place (CIP) cycles, Tangential Flow Filtration (TFF), and chromatography operations.
- Computerization: Which aspects of your production are computerized, and how much repetitive work is still being done on paper?
- Connectivity: How does data flow between teams? Are your IT and OT systems in sync? How are your OT and IT security policies coordinated? Do you have a DMZ for data interconnectivity?
- Visibility: Do you have real-time visibility of your processes and their performance?
- Transparency: How comprehensive, precise, and open are your records? This steps is where you can get more advanced insights into your data and find opportunities to improve!
- Predictability: Are you able to correct problems before they happen?
- Adaptability: Are you adaptable to market demand change?
Filling and Packaging
Continuous monitoring of variables in the filling and packaging process, such as fill weight, seal integrity, and labeling accuracy, can help ensure accurate dosing, proper sealing, and correct labeling of pharmaceutical products.
Package and Seal integrity are critical in this industry, and many manufacturers use human operators to verify package integrity and sterility, which is labor-intensive and can be inconsistent at times. A deep-learning vision-based system can capture high-resolution images of the sealed packages and products, and analyze them for any irregularities, such as gaps, wrinkles, or improper alignment. By comparing the captured images to reference images of properly sealed packages, the vision system can identify potential seal defects.
Labeling accuracy: A vision system can scan labels on packaged pharmaceutical products and verify their accuracy and placement. It can read the text, verify barcodes or QR codes, and check for alignment and legibility. By comparing the scanned information with the expected labeling specifications, the vision system can identify any errors or discrepancies. This helps prevent mislabeling, incorrect dosage instructions, or product mix-ups.
Predictive Maintenance in Pharmaceutical Manufacturing
Over 80% of equipment failures in manufacturing are caused by poor operating conditions or human error, rather than wear and tear over time. The pharmaceutical industry heavily depends on the quality and accuracy of the manufacturing equipment. The failure of vital equipment such as bioreactors, which are essential to produce biopharmaceuticals, vaccines, and other biological products, could result in a loss of over $30,000 per batch. AI-powered predictive maintenance monitors your equipment at all times and helps prevent costly unplanned downtime and lost production. The AI algorithm learns the normal operating patterns of equipment by collecting data on variables such as temperature, pressure, and agitation speed, and will predict failures before they occur.
Examples of other equipment that could hugely benefit from this technology include tablet compression machines and chromatography systems.
The future of biopharma lies in the integration of Industry 4.0 technologies such as AI and ML. Bridge Automation stands at the forefront of this transformative era, capable of revolutionizing pharmaceutical development, manufacturing, and delivery. Join us on this journey as we unlock new possibilities, drive innovation, and shape the future of healthcare together.
To learn more about our solutions, contact Billy Few, principal, at Bridge Automation.
get connected









